Reaching Patients Through
Immunology Innovation
The science of co-creation drives our quest to engineer life-changing immunology solutions, the resilient spirit of patients fuels our urgency to deliver them.
The infinity sign symbolizes our commitment to science and patients; it has no bounds.

Patients

Innovation
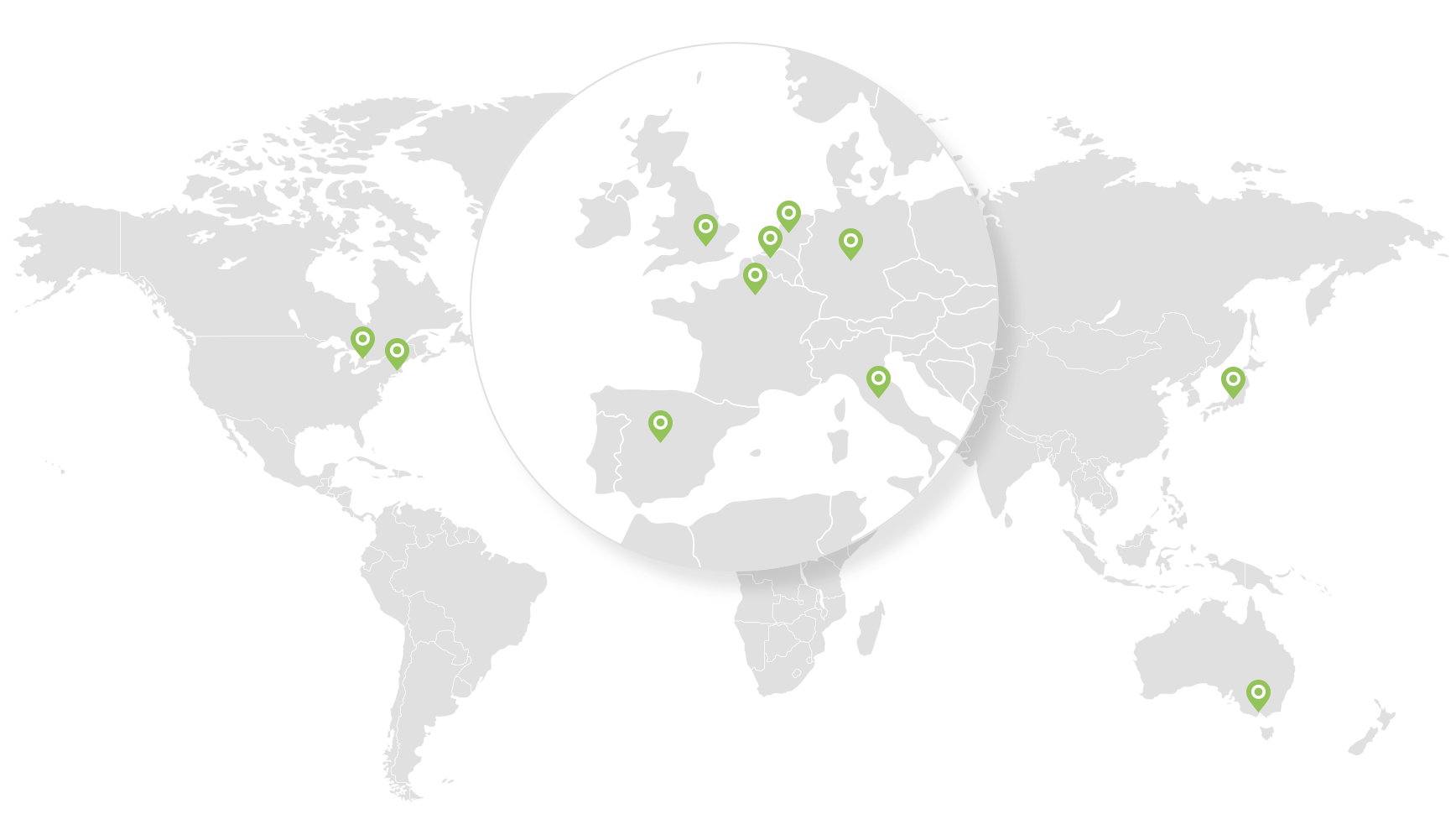
Where we operate
Together We Discover
We know that leaps in progress will come from collaboration and incorporating innovation into every step. We embrace the power of the collective – together we are better.
Our Discoveries
At argenx, we’ve created an antibody innovation ecosystem where pioneering scientists and antibody engineers work side-by-side to accelerate the discovery of novel targets, disease pathways and differentiated therapeutic antibodies.
Pipeline
Product Candidates
Our proprietary portfolio is focused on first-in-class product candidates engineered in collaboration with our academic partners to help translate immunology breakthroughs into medicines.
Our Pride
Efgartigimod
FcRn
We are investigating efgartigimod for the treatment of several severe autoimmune diseases. Efgartigimod is marketed as VYVGART and VYVGART Hytrulo for the treatment of generalized myasthenia gravis.
Designed to block FcRn and reduce IgGs, efgartigimod could be a rational approach to treating diseases where IgG antibodies are pathogenic or disease-causing.
ARGX-117
C2
We are evaluating ARGX-117 for the treatment of severe IgM driven auto-immune diseases.
ARGX-117 is designed to be a humanized sweeping antibody intended to inhibit the function of C2 and downstream complement activation.