Empasiprubart
Empasiprubart (formerly ARGX-117) is designed to be a humanized sweeping antibody that binds specifically to C2 in a pH- and Ca2+- dependent manner. C2 is a protein in the complement cascade which, when activated, leads to cell destruction. Binding of Empasiprubart is intended to inhibit the function of C2 and downstream complement activation.
By blocking complement activity, Empasiprubart has the potential to reduce tissue inflammation and the adaptive immune response. Through this proposed mechanism, Empasiprubart could represent a broad pipeline opportunity across severe autoimmune indications.
- ARGX-117 is designed to block complement activity with potential to reduce tissue inflammation and the adaptive immune response
- IV ARGX-117 and SC ARGX-117 (enabled with Halozyme's ENHANZE® drug delivery technology)
Proposed Mechanism
Of Action
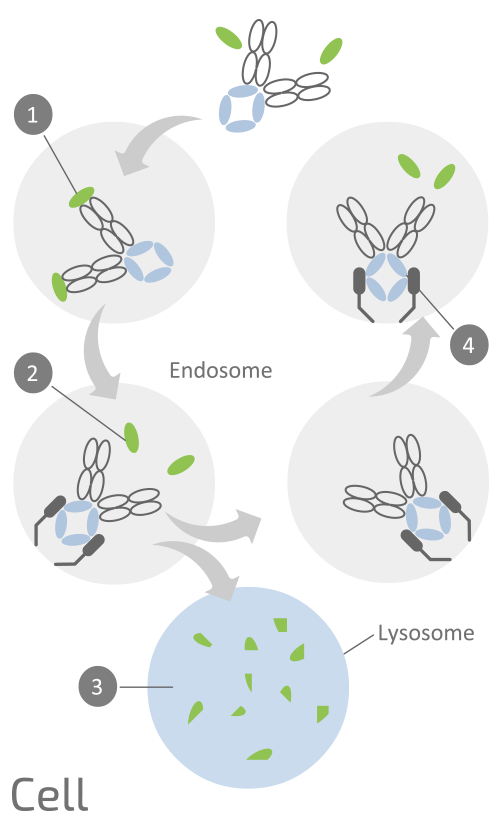
Empasiprubart is engineered as a human IgG1 anti-C2 “sweeping” antibody. Generally, serum proteins like antibodies, are transported from the serum into the cell’s endosome then to the lysosome where they are degraded. Empasiprubart is designed with an Fc backbone that is equipped with the proprietary NHance™ mutation, which is intended to enhance the binding of the antibody to FcRn in the endosome and prevent the antibody going into the lysosome for degradation.
(1) Empasiprubart binds at its variable domain to the target C2 with high affinity in the serum but lower affinity in the endosome due to the pH and Ca2+ dependent nature of the binding.
(2) The lower affinity allows C2 to dissociate from Empasiprubart in the endosome and cycle into the lysosome for destruction (3).
(4) Empasiprubart still bound to FcRn can cycle back to circulation and bind new C2 – repeating the cycle in a “sweeping” manner to continuously destroy the C2 target. With sweeping recycling properties, it is expected that Empasiprubart will remain longer in circulation and can remove several C2 molecules from circulation resulting in enhanced C2 clearance from the bloodstream.